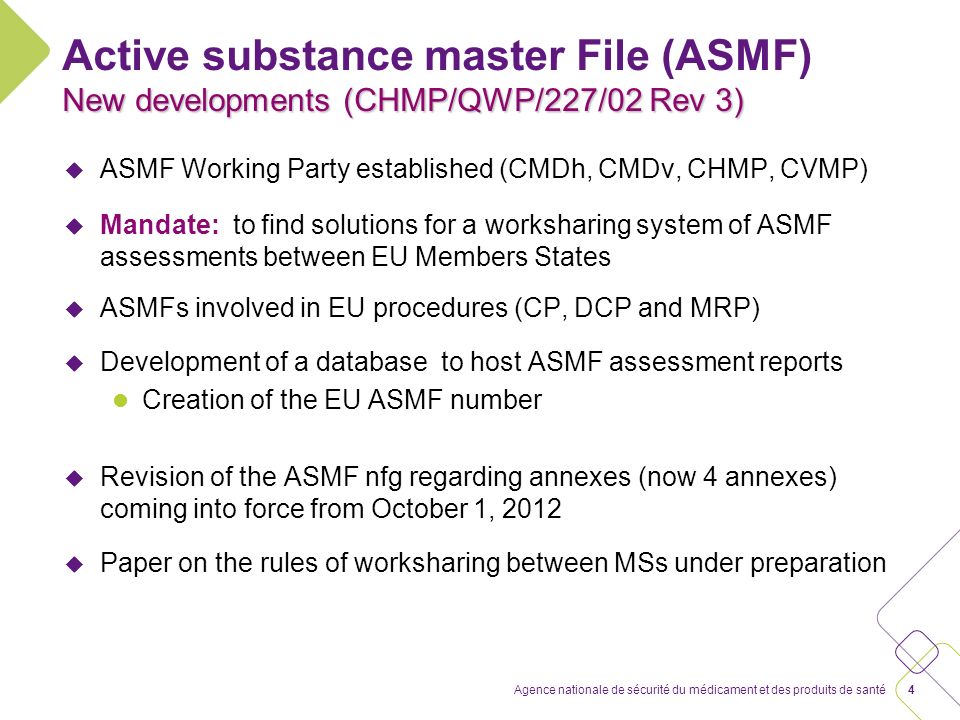
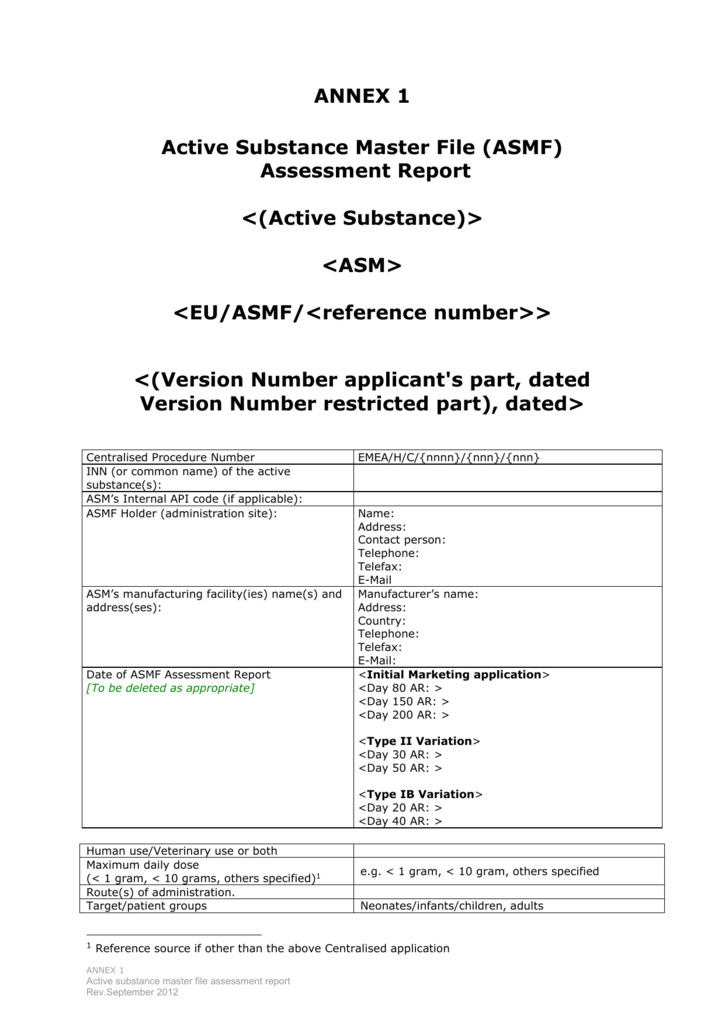
hoffmann6383 on Twitter: "$NWBO Remember the Master File that LP spoke about at the ASM? It should be completed. MAA submission anytime. https://t.co/CSxldp4G4l" / X

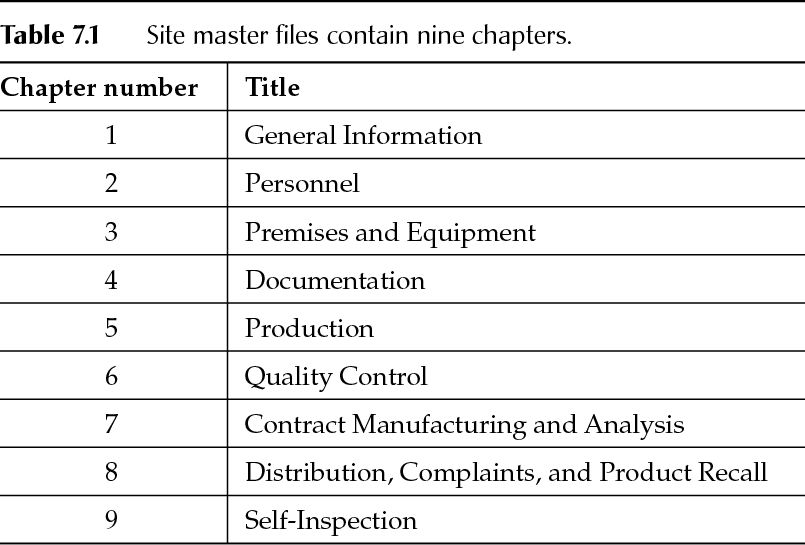
Dr. Raghuveer Pharma Consultants D R P C Quality. Perfection. Confidentiality. D R P C Quality. Perfection. Confidentiality ppt download

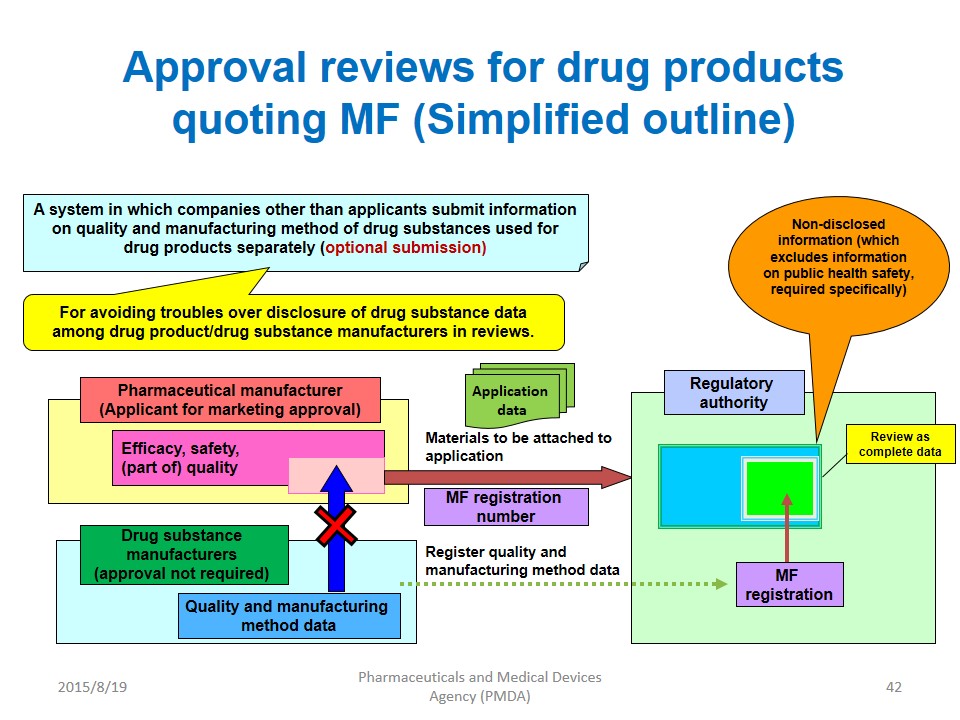
DMF Procedures and Communication between API, FFP Manufacturers and Regulatory Authorities Jean-Louis ROBERT National Health Laboratory L – 1011 LUXEMBOURG. - ppt download